|
Today I am starting a multiple part “What the Hell?” series on the subject of Cancer. There are many different aspects of cancer biology from what cancer is to how we diagnose and treat cancer and how we can prevent cancer. These posts will be a bit longer than usual so bear with me. I want to cover all of these subjects in enough detail that you as a reader can walk away with a few answers and maybe also a few questions.
HEALTH WARNING: These posts will be long. There is a lot of text. Feel free to read a little and come back. To begin: If you don’t take anything away from these posts, I do want you to take away one VERY important thing. Like all facets of life, cancer is not black and white but a vast, daunting expanse of grey. As unique you are from the next person, cancer is unique to the person who has it. While cancers fall into “families” based on location or genetic similarities (e.g. breast, lung, etc.), each one has it’s own characteristics that make it unlike other cancers in the same “family”. In the simplest way I can come up with, cancer is a collection of your own cells that have gone mad. And that madness is unique to you.
Cancer classification
1. Solid Tumours:
Blood (liquid) Cancer:
Terminology:
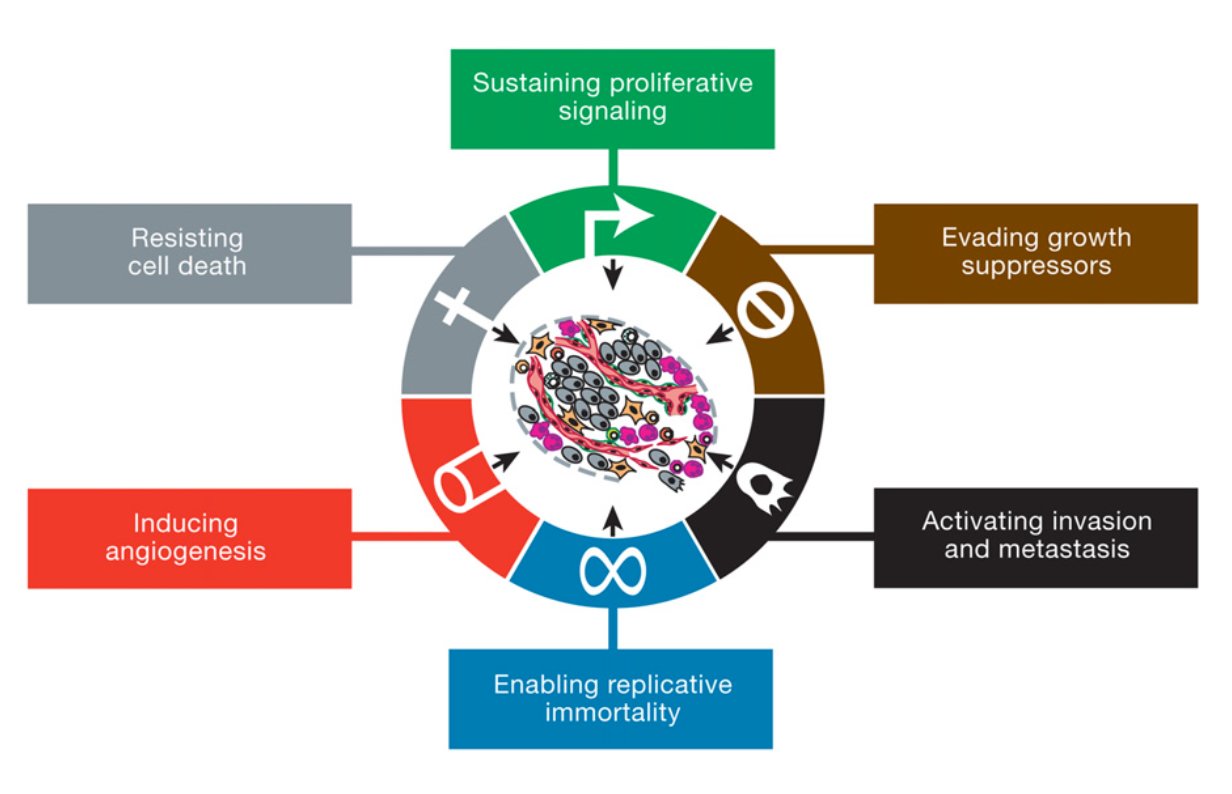
As I said cancer is your own cells that have gone mad. In more scientific terms, there a certain number of characteristics (or "Hallmarks") that define cancer set out by Hanahan and Weinberg originally in 2000 and later updated in 2011. Figure 1 shows the classic “Hallmarks of Cancer”.
The first three Hallmarks are very related and focus around signalling. In a nutshell, cancer cells need to continue to grow while preventing the cancer cells from dying.
(1) Sustaining proliferative signalling Normal cells carefully balance growing with not growing. This process maintains the right number of functioning cells. Growth signals are given when more cells are needed but are shut off when no cells are needed. In cancer, the growth signals are constantly active. This means the cells are constantly growing and dividing. They can do this in a few ways:
(2) Evading growth suppressors As I’ve said before growth is tightly regulated. There are lots of signals, which inhibit the growth of cells. Cancer cells actively prevent these signals from preventing growth. This can be done by:
(3) Resisting Cell Death Cancer cells do not want to die. They want to keep growing and dividing. Normal cells have a few ways of dying which controls cell numbers but also prevents cells with DNA damage or bad mutations from becoming cancerous. These pathways include: apoptosis (programmed cell death), necrosis (un-programmed cell death) and autophagy (where the cells breaks down part of their own cell structure to survive). Cells receive internal (DNA damage, shortened telomeres etc.) or external signals (from other cells) which tell the cell "Listen...I think it's time for you to pop your clogs". Cancer cells deregulate cell death pathways by shutting down the pathways that lead to cell death and increasing the pathways that inhibit cell death. This means no matter how many internal or external signals the cells get to die, the cancer cell will ignore them and continue being a cancer cell. In summary for the first three Hallmarks, cancer completely re-wires all the signalling pathways in the cell to promote their growth and division while ignoring any prompts to stop growing and die.
The next three Hallmarks give cancer cells a growth advantage and allows the cells to move around the body.
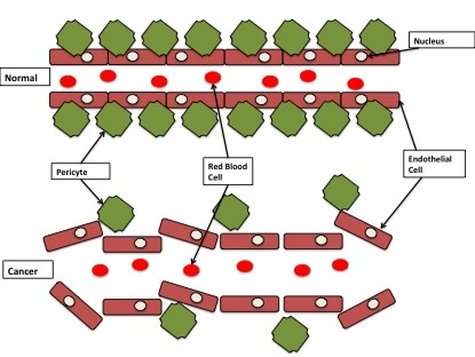
(4) Enabling replicative immortality Cancer cells are immortal. This means if they were allowed to keep going uncontrolled, they will grow and never die. Normal cells have a life cycle. They grow and divide and if they don't receive premature death signals, they keep going until they have a "natural death". This is controlled by things called "telomeres". These are long stretches of "TTAGGG" base repeats that sit on the end of chromosomes, like the caps on the end of your shoe laces. They protect parts of the chromosome from being lost during cell division. Every time a normal cell divides the telomeres shorten a little bit. When they become too short the cell reaches a "crisis" and dies. This is a natural death for a cell. As a cancer cell starts to become malignant, the telomeres still shorten. However when the telomeres reach "crisis", the cancer cells turn on an enzyme called "telomerase". Instead of losing telomere repeats during every cell division, telomerase now adds telomere repeats. This essentially prevents the cells from going through natural death and makes them immortal. (5) Inducing angiogenesis All your cells need a healthy blood supply. Blood vessels bring oxygen and nutrients to cells and take away all the bad rubbish like CO2 and waste. Your organs are covered in blood vessels to make sure every cell has access. When your cells have a good supply of oxygen it is called "normoxia" (basically normal oxygen). When your cells are starved of oxygen it is called "hypoxia". You don't want your cells to become hypoxic because it kills cells. As a tumour develops it wants to grow near a good blood supply. But that may not be enough so cancer cells can grow their own blood vessels. This can be part of the early development of the tumour or because the tumour is so big the cells in the middle are all hypoxic. Cancer cells grow blood vessels by sending out messages to their local blood vessels (for example VEGF-A or FGF). This causes the blood vessel cells (endothelial cells) to branch off from the main blood vessel and grow towards the tumour. Tumour blood vessels are pretty badly made (figure 3). They're leaky and are pretty weak with large gaps between cells instead of a smooth blood vessel. While inefficient, they work well enough for the tumour to get the oxygen and nutrients it needs.
(6) Activating Invasion and Metastasis
The final established Hallmark of cancer is the ability of cancer cells to spread. Invasion: Cells live together in organs and tissues. But there is a lot of stuff outside the cells which provide support and structure for the cells (for example collagen). This is called the "extracellular matrix (ECM)". The ECM is pretty stiff. This prevents cells from moving around too much, giving them an anchor to other cells. In cancer, cells want to occupy the area around them. Essentially they "invade" into the extracellular matrix. They do this by:
Metastasis:
Invasion and angiogenesis (making blood vessels) allow cancer cells to metastasise. Metastasis is basically cancer cells moving from one part of the body to another. The primary tumour (the original tumour) forms a secondary tumour (the new tumour) in a different part of the body. The old tumour and new tumour share very similar characteristics. Cancer cells usually don't just move anywhere but pick specific parts of the body to call home based on the preferences of the primary tumour. For example Breast Cancer usually spreads into the chest cavity, liver and brain. How cancer cells pick the new sites is relatively unknown. Once the cancer cells invade into the ECM they can move towards blood vessels. When they're in the blood stream they surround themselves with immune cells. This is the not only to avoid being killed by the immune system but also to withstand the pressure of blood vessels. If the cancer cell did not protect itself it would be torn apart very quickly. The cancer cells can travel vast distances throughout the body. When a cancer cell picks a site for the secondary tumour, it leaves the blood and starts to break down the ECM at the new site to create space to grow. The cancer cells also transforms back into epithelial cells (MET). Even though primary tumours are constantly shedding cancer cells, only 0.1% of cancer cells broken off from a primary tumour actually form secondary cancers. 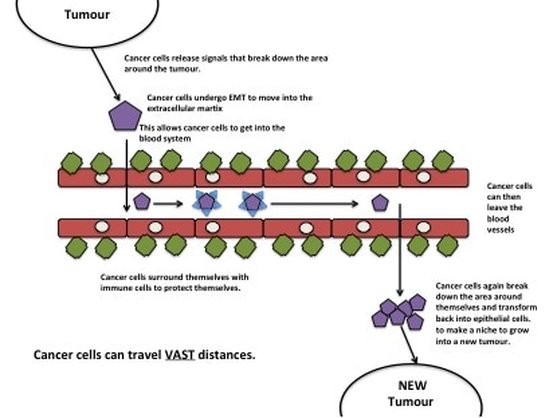
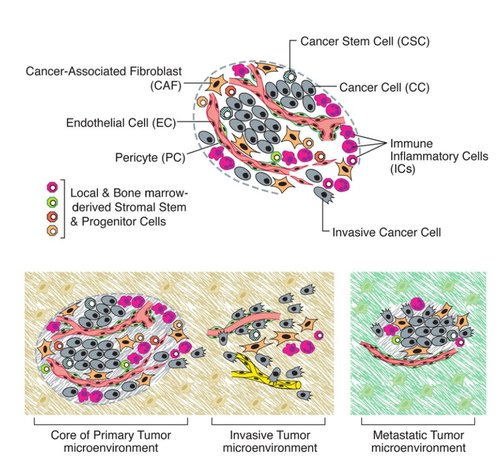
Figure 4: Tumours release signals into the microenvironment to create a path for an individual cancer cell (purple cell) to get to blood vessels. Once in the blood vessel, the cancer cell hijacks immune cells to protect itself (blue triangles). The cancer cell can travel vast distances all around the body. The cancer cell then leaves the blood vessel. It breaks down the microenvironment around itself to create a space to grow into a new tumour.
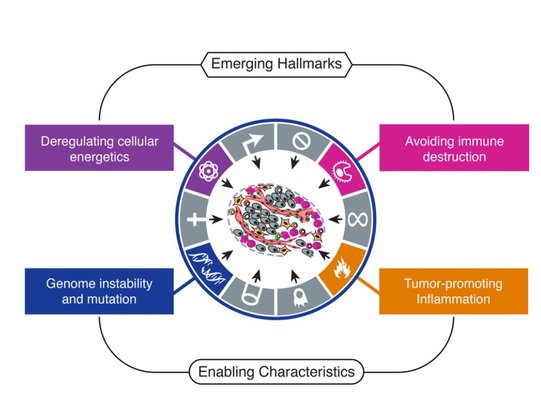
In 2011, Hanahan and Weinberg updated their Hallmarks with four more, two Emerging Hallmarks and two Enabling Hallmarks.
Emerging Hallmarks
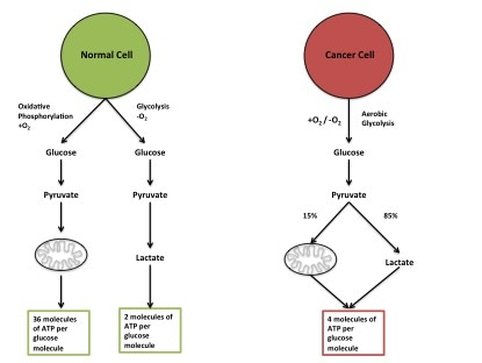
(1) Deregulating Cancer Energetics: Cancer cells use a lot of energy compared to normal cells. This is because they are constantly growing and creating proteins, lipids and nucleic acids. The primary source of energy cancer cells use is glucose. To get the most bang for their buck cancer cells do something called the "Warburg Effect" (figure 6). In normal cells energy is made using "oxidative phosphorylation". While you get a lot of energy per glucose molecule, it takes a long time. Whereas another form of energy making, "glycolysis", is extremely quick. You don't get as much energy but cancer cells are more concerned with speed than efficiency. Glucose can also run out quite quickly so cancers have adapted to use other sources of energy for example lactate.
(2) Avoiding Immune Destruction:
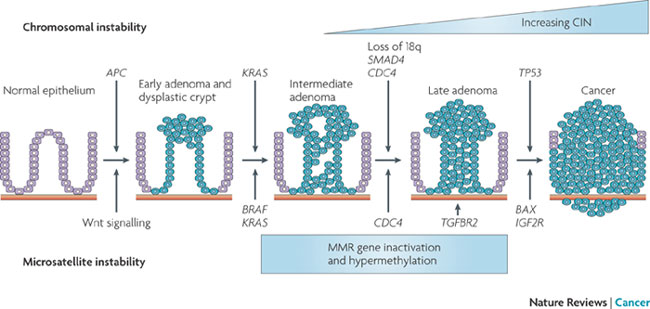
Your immune system is pretty sensitive to foreign invaders. All cells have signals on their cell surfaces (called antigens). Immune cells produce antibodies which bind to these signals. If the signal comes from your own normal cells, the immune cell leaves it alone (kind of like a friendly handshake). If the immune system doesn't recognise the antigen then it attacks the cell. Cancer cells are not normal and do express antigens which immune cells do not recognise. To avoid being destroyed by immune cells, cancer cells express the "normal" antigens to trick the immune cells. Cancer cells can also hijack the immune system. By sending signals to certain immune cells they can transform them into pro-cancer immune cells. When an anti-cancer immune cell tries to destroy the cancer cell, the pro-cancer tumour cell comes in (kind of like a body guard) and suppresses the anti-cancer immune response. Enabling Hallmarks: (1) Genome Instability and Mutation I think I've established that cancer cells have lots of mutations which helps them grow and divide and generally be cancer. But there are certain mutations and genome instability events that are indicative of certain cancers. Cancers go though a transformation pathways from normal cells to malignant to metastasis. Each type of cancer has a sequence of events that gets them to that stage. For example figure 7 shows this sequence in Colorectal Cancer.
(2) Tumour-Promoting Inflammation
Inflammation occurs naturally in the body, usually in response to an injury. Inflammation is the response of the immune system to injury or damage. As I've said before, cancer cells hijack the immune system to protect themselves. But a side effect of this is the immune cells create a highly pro-cancer environment. Inflammation leads to the release of a wide range of growth signals, it promotes new blood vessels to grow amongst other things. In essence inflammation is the perfect environment for a tumour to grow.
So those are the main Hallmarks of Cancer. While these were defined in 2000 and 2011, how cancer cells do any of these processes is still largely a mystery. I will talk later about how we can target Hallmarks of Cancer to kill cancer.
0 Comments
Leave a Reply. |
AuthorMy name is Caitriona and I am a PhD student at Imperial College London, UK. Categories
All
|