|
In this edition of "What the Hell? - Cancer" I will going through how cancers are diagnosed. This will hopefully give you an idea about what the different stages of cancer are and how each cancer is graded differently.
NOTE: All my examples will be using breast cancer because it is the cancer type I study and am most familiar with. Before I begin I want to just clear up the differences between primary tumours, secondary tumours and primary secondary tumours.
Cancer diagnosis is a tricky business. If you read my awareness posts you will see the signs and symptoms of a lot of different cancers. You may have noticed that a lot of the signs and symptoms are generic for example bloating, intestinal discomfort, a prolonged cough etc.
Detection
Tumours need to be detected before you can take a biopsy etc. These are the main ways tumours are detected (but not the only ways). Apologies for the explanations. While I learnt all this in my masters (woo nuclear physics) I am no physicist...
Pathology
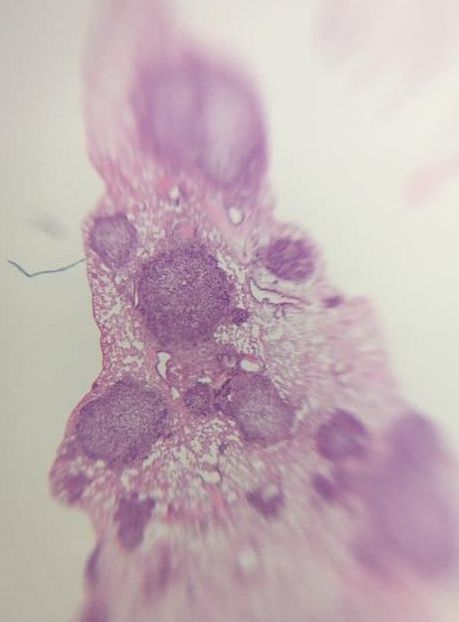
Part of what pathologists are looking for when they stain biopsies is structural changes to cells. Cells "adapt" to their environment or from internal cues by changing their structure. There are a number of cellular adaptions which happen normally but can also be due to disease etc.
The next three are characteristic of pre-cancer cells (i.e. structural changes that can lead to cancer):
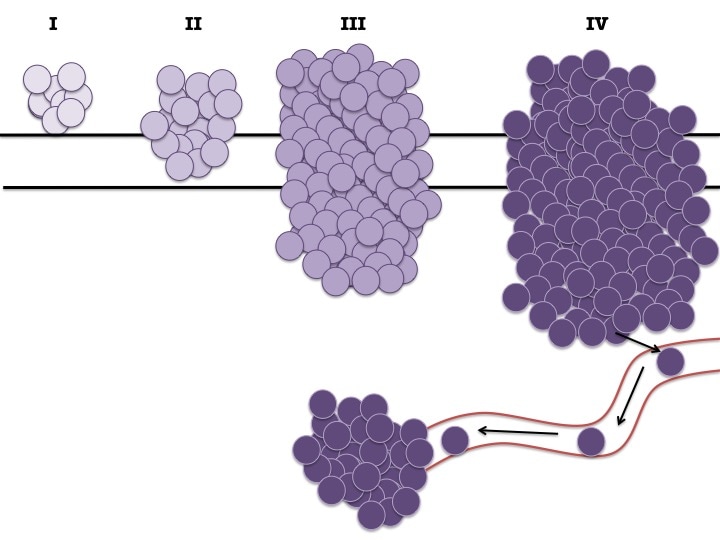
Change of shape and structure and the number of darkly stained cells allow pathologists to grade tissue samples. Histopathology is based very much on the person and takes years and years to generate enough knowledge to be able to look down a microscope at a purple stained piece of tissue and say "that is a squamous cell carcinoma" (type of skin cancer - don't look up images). There is a lot more to the work of a pathologist but I don't have the knowledge to delve deeper. Staging and Grading
So what do I mean by stage? Well the stage of the cancer tells you (in general terms) how big the tumour is, if it has invaded locally or systemically and what treatment you should use.
Clinicians expand on this and use a system called TNM. This is "Tumour", "Node", "Metastasis".
As I said before each cancer type has slightly different staging criteria. This is due to the type of organ it is in (some organs are smaller than others so a "small" tumour in a large organ is actually quite a big tumour in a smaller organ). The staging also has to take into account where the tumour invades locally and systemically and if lymph nodes are involved.
In breast cancer:
Other cancers like Colorectal Cancer use additional staging techniques for example Duke's Staging which is very like the numbered system but is marked by grade A-D. Location/Origin
But the above is not the only way to identify the type of cancer! Oh no, like all aspects of cancer biology there is always more.
While the stage/grade will help you with treatment options there are other factors such as location that tell you about the cancer. For example in breast cancer: Epithelial Tumours:
Other:
Mutations and Chromosomal Changes
And as always we come back to DNA. There are mutations that are common in pretty much every cancer type (e.g. p53) but other mutations are specific to certain cancers of families of cancers. For example BRCA mutations are associated with both breast and ovarian cancers.
In breast cancer the common mutations used to define cancer types are:
In breast cancer if you have suspected Her2+ cancer a number of tests are done such as Immunohistochemistry (IHC) which detects Her2 receptor levels on the surface of the breast cancer cell and Fluorescence In Situ Hybridisation (FISH) which detects the number of copies of the HER2 gene there are. There are genetic screening tests used in the clinic for some cancers. In breast cancer there is OncotypeDx which detects 21 genes commonly mutated in breast cancer. This gives you a score. OncotypeDx also has systems for prostate and colon cancer. Changes to the chromosomes themselves are also common, usually in haematological malignancies.
In chronic myelogenous leukaemia (CML), there is a translocation called BCR-ABL where part of the chromosome where the ABL gene is located (Chr.9) breaks off and switches with the part of the chromosome where BCR is located (Chr.22). This brings BCR and ABL together creating a powerful oncogene.
And you combine them all. You take your image of the tumour(s) with all of the measurements and origin of the primary tumour, you get the pathologists report from the biopsy about the type of cancer it's believed to be, how fast it's growing, what proteins it may be expressing on the cell surface and how aggressive it is and lastly genetic testing to determine the mutations. This gives you a picture which allows you to provide the best treatment plan for that patient.
So a patient could have a T2N1M0 ER+ invasive lobular breast carcinoma, meaning they have a breast cancer which originated from the epithelial cells in the lobes of the breast, the cells show structural changes and it is between 2cm and 5cm across, there 1-2 lymph nodes with infiltrating breast cells, there is no metastasis and the tumour is oestrogen receptor positive. The treatment path for this patient may be surgery to remove the tumour and lymph nodes involved or a full mastectomy. Depending on the surgery, the patient may receive chemotherapy and will more than likely be given tamoxifen (anti-oestrogen receptor drug) for 5-10 years. It's difficult to describe every type of cancer's staging systems because as I said it really is dependent on the type of tumour.
And that is the basics of it. As always if you have questions please don't hesitate to ask. This isn't all of the information and if you or someone you know is affected by cancer they should ask their attending physician to explain their diagnosis to them (cancer is so nuanced).
Hopefully in the next month or so I will put up our penultimate "What the Hell? - Cancer" blog post on Cancer Treatment! For more information check out CRUK's website https://www.cancerresearchuk.org/
0 Comments
Today I am starting a multiple part “What the Hell?” series on the subject of Cancer. There are many different aspects of cancer biology from what cancer is to how we diagnose and treat cancer and how we can prevent cancer. These posts will be a bit longer than usual so bear with me. I want to cover all of these subjects in enough detail that you as a reader can walk away with a few answers and maybe also a few questions.
HEALTH WARNING: These posts will be long. There is a lot of text. Feel free to read a little and come back. To begin: If you don’t take anything away from these posts, I do want you to take away one VERY important thing. Like all facets of life, cancer is not black and white but a vast, daunting expanse of grey. As unique you are from the next person, cancer is unique to the person who has it. While cancers fall into “families” based on location or genetic similarities (e.g. breast, lung, etc.), each one has it’s own characteristics that make it unlike other cancers in the same “family”. In the simplest way I can come up with, cancer is a collection of your own cells that have gone mad. And that madness is unique to you.
Cancer classification
1. Solid Tumours:
Blood (liquid) Cancer:
Terminology:
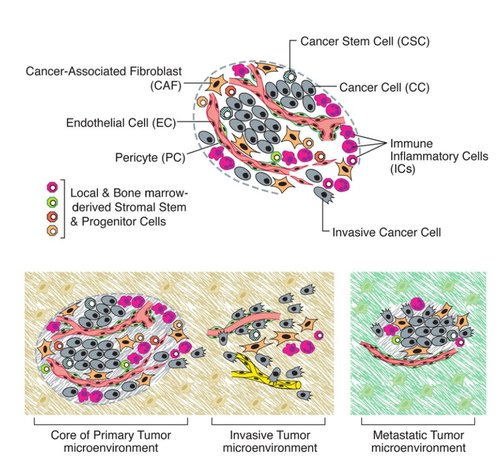
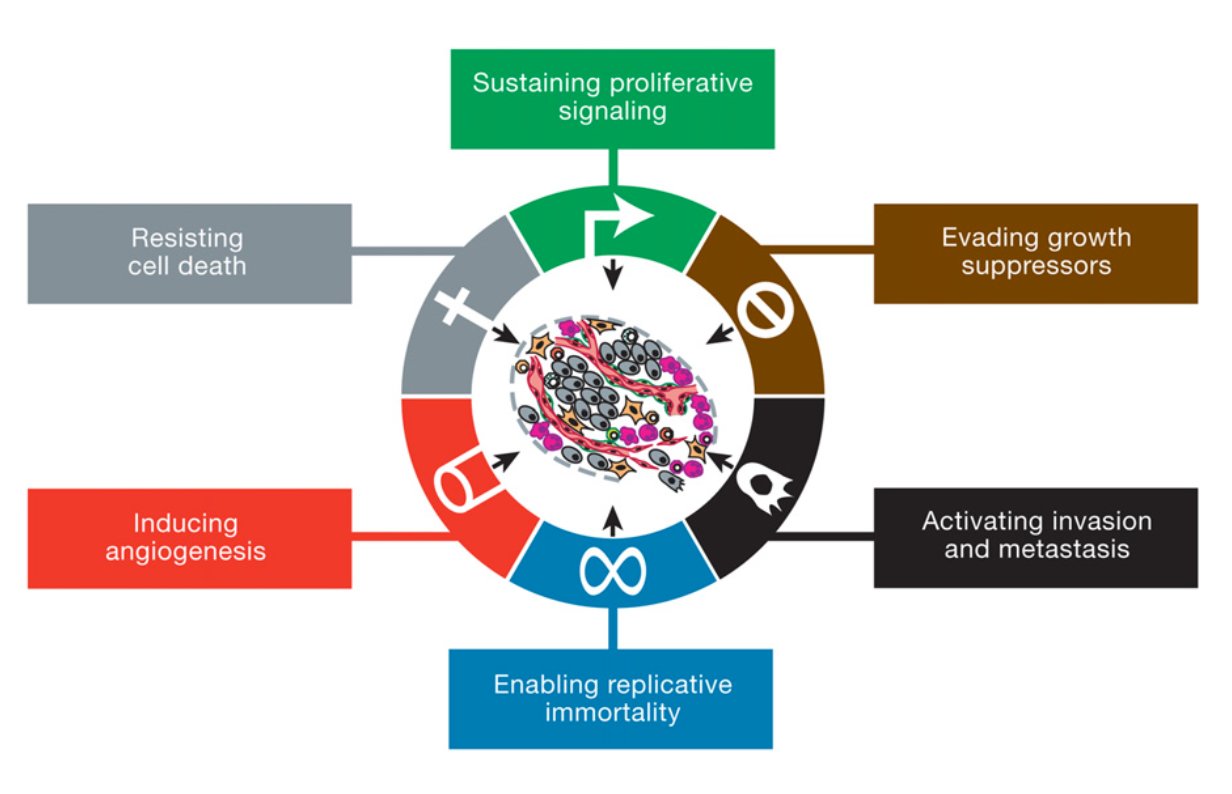
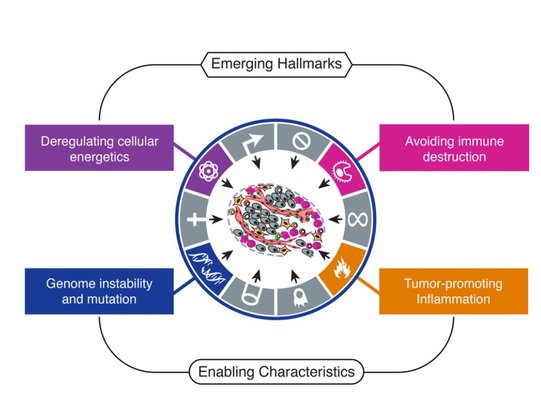
As I said cancer is your own cells that have gone mad. In more scientific terms, there a certain number of characteristics (or "Hallmarks") that define cancer set out by Hanahan and Weinberg originally in 2000 and later updated in 2011. Figure 1 shows the classic “Hallmarks of Cancer”.
The first three Hallmarks are very related and focus around signalling. In a nutshell, cancer cells need to continue to grow while preventing the cancer cells from dying.
(1) Sustaining proliferative signalling Normal cells carefully balance growing with not growing. This process maintains the right number of functioning cells. Growth signals are given when more cells are needed but are shut off when no cells are needed. In cancer, the growth signals are constantly active. This means the cells are constantly growing and dividing. They can do this in a few ways:
(2) Evading growth suppressors As I’ve said before growth is tightly regulated. There are lots of signals, which inhibit the growth of cells. Cancer cells actively prevent these signals from preventing growth. This can be done by:
(3) Resisting Cell Death Cancer cells do not want to die. They want to keep growing and dividing. Normal cells have a few ways of dying which controls cell numbers but also prevents cells with DNA damage or bad mutations from becoming cancerous. These pathways include: apoptosis (programmed cell death), necrosis (un-programmed cell death) and autophagy (where the cells breaks down part of their own cell structure to survive). Cells receive internal (DNA damage, shortened telomeres etc.) or external signals (from other cells) which tell the cell "Listen...I think it's time for you to pop your clogs". Cancer cells deregulate cell death pathways by shutting down the pathways that lead to cell death and increasing the pathways that inhibit cell death. This means no matter how many internal or external signals the cells get to die, the cancer cell will ignore them and continue being a cancer cell. In summary for the first three Hallmarks, cancer completely re-wires all the signalling pathways in the cell to promote their growth and division while ignoring any prompts to stop growing and die.
The next three Hallmarks give cancer cells a growth advantage and allows the cells to move around the body.
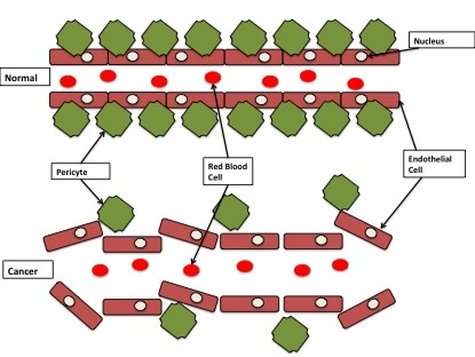
(4) Enabling replicative immortality Cancer cells are immortal. This means if they were allowed to keep going uncontrolled, they will grow and never die. Normal cells have a life cycle. They grow and divide and if they don't receive premature death signals, they keep going until they have a "natural death". This is controlled by things called "telomeres". These are long stretches of "TTAGGG" base repeats that sit on the end of chromosomes, like the caps on the end of your shoe laces. They protect parts of the chromosome from being lost during cell division. Every time a normal cell divides the telomeres shorten a little bit. When they become too short the cell reaches a "crisis" and dies. This is a natural death for a cell. As a cancer cell starts to become malignant, the telomeres still shorten. However when the telomeres reach "crisis", the cancer cells turn on an enzyme called "telomerase". Instead of losing telomere repeats during every cell division, telomerase now adds telomere repeats. This essentially prevents the cells from going through natural death and makes them immortal. (5) Inducing angiogenesis All your cells need a healthy blood supply. Blood vessels bring oxygen and nutrients to cells and take away all the bad rubbish like CO2 and waste. Your organs are covered in blood vessels to make sure every cell has access. When your cells have a good supply of oxygen it is called "normoxia" (basically normal oxygen). When your cells are starved of oxygen it is called "hypoxia". You don't want your cells to become hypoxic because it kills cells. As a tumour develops it wants to grow near a good blood supply. But that may not be enough so cancer cells can grow their own blood vessels. This can be part of the early development of the tumour or because the tumour is so big the cells in the middle are all hypoxic. Cancer cells grow blood vessels by sending out messages to their local blood vessels (for example VEGF-A or FGF). This causes the blood vessel cells (endothelial cells) to branch off from the main blood vessel and grow towards the tumour. Tumour blood vessels are pretty badly made (figure 3). They're leaky and are pretty weak with large gaps between cells instead of a smooth blood vessel. While inefficient, they work well enough for the tumour to get the oxygen and nutrients it needs.
(6) Activating Invasion and Metastasis
The final established Hallmark of cancer is the ability of cancer cells to spread. Invasion: Cells live together in organs and tissues. But there is a lot of stuff outside the cells which provide support and structure for the cells (for example collagen). This is called the "extracellular matrix (ECM)". The ECM is pretty stiff. This prevents cells from moving around too much, giving them an anchor to other cells. In cancer, cells want to occupy the area around them. Essentially they "invade" into the extracellular matrix. They do this by:
Metastasis:
Invasion and angiogenesis (making blood vessels) allow cancer cells to metastasise. Metastasis is basically cancer cells moving from one part of the body to another. The primary tumour (the original tumour) forms a secondary tumour (the new tumour) in a different part of the body. The old tumour and new tumour share very similar characteristics. Cancer cells usually don't just move anywhere but pick specific parts of the body to call home based on the preferences of the primary tumour. For example Breast Cancer usually spreads into the chest cavity, liver and brain. How cancer cells pick the new sites is relatively unknown. Once the cancer cells invade into the ECM they can move towards blood vessels. When they're in the blood stream they surround themselves with immune cells. This is the not only to avoid being killed by the immune system but also to withstand the pressure of blood vessels. If the cancer cell did not protect itself it would be torn apart very quickly. The cancer cells can travel vast distances throughout the body. When a cancer cell picks a site for the secondary tumour, it leaves the blood and starts to break down the ECM at the new site to create space to grow. The cancer cells also transforms back into epithelial cells (MET). Even though primary tumours are constantly shedding cancer cells, only 0.1% of cancer cells broken off from a primary tumour actually form secondary cancers. 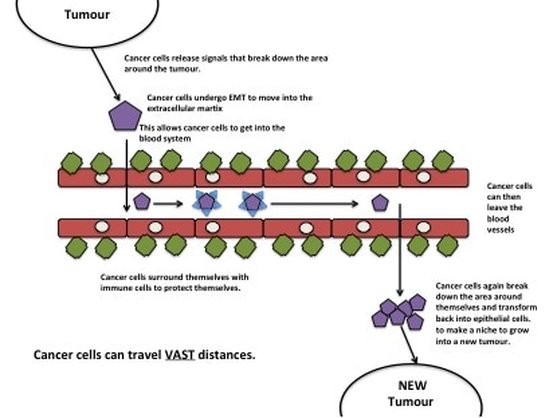
Figure 4: Tumours release signals into the microenvironment to create a path for an individual cancer cell (purple cell) to get to blood vessels. Once in the blood vessel, the cancer cell hijacks immune cells to protect itself (blue triangles). The cancer cell can travel vast distances all around the body. The cancer cell then leaves the blood vessel. It breaks down the microenvironment around itself to create a space to grow into a new tumour.
In 2011, Hanahan and Weinberg updated their Hallmarks with four more, two Emerging Hallmarks and two Enabling Hallmarks.
Emerging Hallmarks
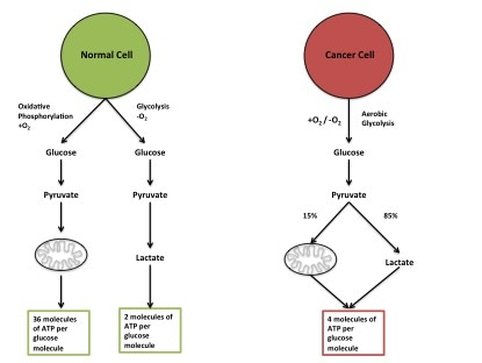
(1) Deregulating Cancer Energetics: Cancer cells use a lot of energy compared to normal cells. This is because they are constantly growing and creating proteins, lipids and nucleic acids. The primary source of energy cancer cells use is glucose. To get the most bang for their buck cancer cells do something called the "Warburg Effect" (figure 6). In normal cells energy is made using "oxidative phosphorylation". While you get a lot of energy per glucose molecule, it takes a long time. Whereas another form of energy making, "glycolysis", is extremely quick. You don't get as much energy but cancer cells are more concerned with speed than efficiency. Glucose can also run out quite quickly so cancers have adapted to use other sources of energy for example lactate.
(2) Avoiding Immune Destruction:
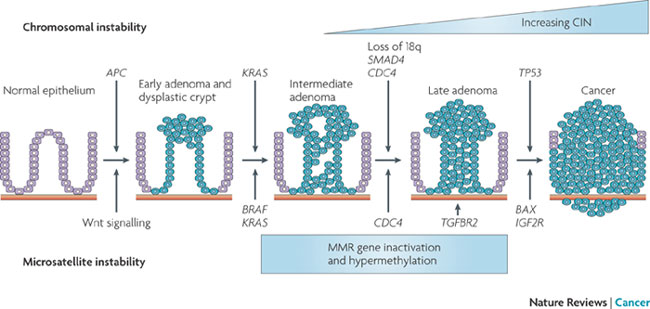
Your immune system is pretty sensitive to foreign invaders. All cells have signals on their cell surfaces (called antigens). Immune cells produce antibodies which bind to these signals. If the signal comes from your own normal cells, the immune cell leaves it alone (kind of like a friendly handshake). If the immune system doesn't recognise the antigen then it attacks the cell. Cancer cells are not normal and do express antigens which immune cells do not recognise. To avoid being destroyed by immune cells, cancer cells express the "normal" antigens to trick the immune cells. Cancer cells can also hijack the immune system. By sending signals to certain immune cells they can transform them into pro-cancer immune cells. When an anti-cancer immune cell tries to destroy the cancer cell, the pro-cancer tumour cell comes in (kind of like a body guard) and suppresses the anti-cancer immune response. Enabling Hallmarks: (1) Genome Instability and Mutation I think I've established that cancer cells have lots of mutations which helps them grow and divide and generally be cancer. But there are certain mutations and genome instability events that are indicative of certain cancers. Cancers go though a transformation pathways from normal cells to malignant to metastasis. Each type of cancer has a sequence of events that gets them to that stage. For example figure 7 shows this sequence in Colorectal Cancer.
(2) Tumour-Promoting Inflammation
Inflammation occurs naturally in the body, usually in response to an injury. Inflammation is the response of the immune system to injury or damage. As I've said before, cancer cells hijack the immune system to protect themselves. But a side effect of this is the immune cells create a highly pro-cancer environment. Inflammation leads to the release of a wide range of growth signals, it promotes new blood vessels to grow amongst other things. In essence inflammation is the perfect environment for a tumour to grow.
So those are the main Hallmarks of Cancer. While these were defined in 2000 and 2011, how cancer cells do any of these processes is still largely a mystery. I will talk later about how we can target Hallmarks of Cancer to kill cancer.
I wanted to give you a brief insight into some aspects of how cells work. This is a warm up for my next few blog posts about cancer.
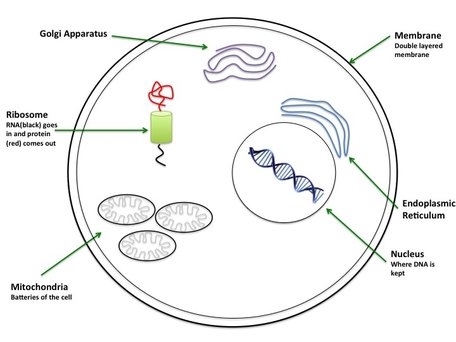
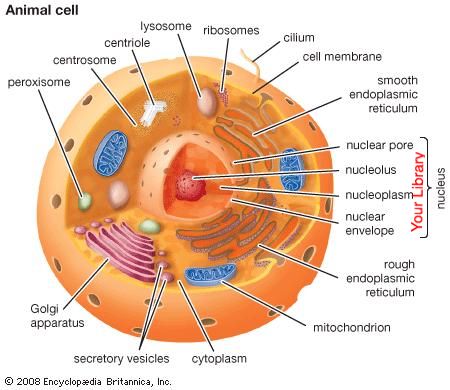
The Cell: The cell is made up a whole lot of things (figure 1 shows just the bare minimum) which keep each cell and your body ticking over. Like a fried egg, the "yolk" of the cell is where all the genetic material is held. There are lots of things that want to degrade DNA in the "white" so DNA is kept in the "nucleus". RNA also spends some time in the nucleus and needs to be protected by "caps" on each end to stop it from damage (like a shoe lace). The "white" or "cytoplasm" is where everything else is. Mitochondria are the batteries for your cell. It is where all your energy is made. You have hundreds of mitochondria to power one tiny cell. Interestingly your mitochondria come completely from your mother. Ribosomes take the RNA made in transcription and translate this into a protein (see What the Hell? - Genetics for more detail). The Endoplasmic Reticulum and Golgi Apparatus are important for the formation of lipids and some proteins and also modifying these proteins for specific functions (e.g. to sit in membranes etc.). The Cell Membrane is a double layered structure that maintains the cell's shape. It also controls very carefully what is allowed enter and exit the cell. For example, your cells need a constant balance of salts (sodium: Na+, potassium: K+ and chloride: Cl- also known as the membrane potential) to maintain ideal cell conditions.
Cell Signalling:
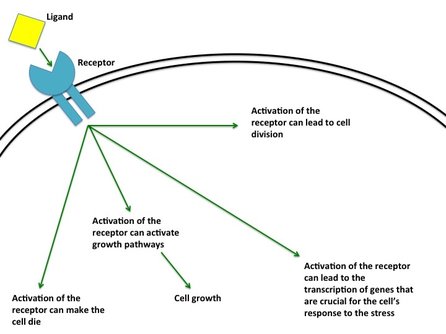
Cell signalling is an incredibly important process for cell survival. It allows cells to communicate to each other as well as letting the cell respond to the external environment. Cell signalling is when a cell responds to a stimulus (either external or internal) leading to a biological process (such as cell division, transcription or cell death). Stimuli include hormones or proteins from other cells. Figure 2 shows external cell signalling in it's simplest form. Imagine you are playing catch with a baseball. A ball (a stimuli known as a ligand) is thrown and is caught by a catcher mitt (known as a receptor). When the ball is caught it "binds" to the mitt. When the receptor on the outer surface of the cell binds to the ligand it sends signal the membrane into the cell. The signal is passed on, activating pathways as it goes or it can be sent back out of the cell. In normal cells, ligands bind specifically to certain receptors. For example oestrogen will bind to the oestrogen receptor.
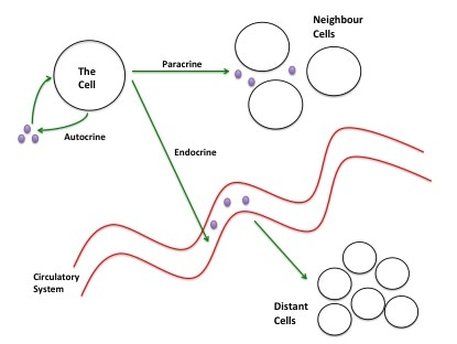
Figure 3 shows three types of cellular signalling:
Cell Division:
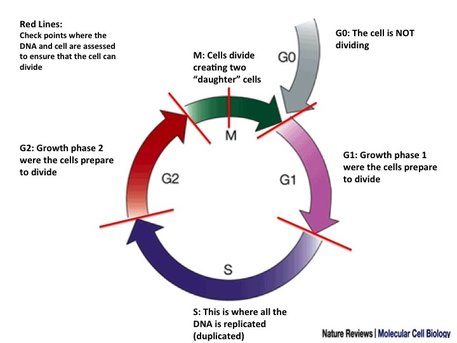
Cell signalling can lead to a very important process in the cell - cell division. Figure 4 shows what happens in cell division. Cell division is crucial for cells to grow and maintain. Cell division allows two "daughter" cells are made from one "parent" cell. The daughter cells are identical to the "parent" cell and each other. This is known as mitosis. Cells are highly complex so duplicating the entire cell structure is a complicated business. Cells go through a number of checks during each "phase" of cell division. It works like a traffic light system, if you have the right conditions and pass all the checks (no bad DNA damage, no bad gene mutations etc.) the cell gets the green light. If there is damage or something like that, the cell gets a yellow until the problem can be fixed. If there is something very wrong or the problems can't be fixed the cell gets a red light. This drives the cell to die. This traffic light system occurs at every check point. Proteins control these check points and the most recognisable cell division check point protein (which you may already know and will definitely hear about A LOT) is p53. One of the things p53 checks for is DNA damage. If the DNA is damaged you do not want the cell dividing. Letting DNA damage pass can lead to DNA mutations or whole chunks of DNA being lost or moving about the genome. A lot of DNA damage can be repaired by a range of different methods but if it can't be repaired p53 pushes the cell to die.
Cell Death:
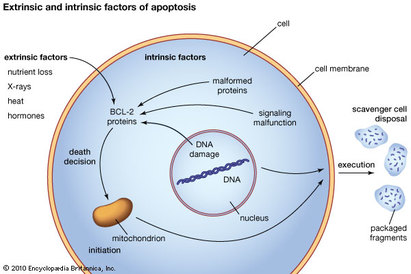
If there is irreversible damage to a cell, a process called apoptosis will be triggered. This process leads to the death of the cell. Figure 5 shows what happens in apoptosis. To begin, a stress signal is detected by the cell. This can be internal, like DNA damage, or external like sudden changes to the cell environment. Other cells can also signal cells to die. Once a death signal is detected this starts a cascade of signals that ultimately leads to the cell breaking up into fragments which are then taken up by neighbouring cells or excreted from the body. The number of cells in your body remain quite constant so when new cells are made, older cells die. This prevents DNA damage and bad mutations from persisting in the body.
These are just a few processes in the cell which will help me explain what cancer is and how we can treat/prevent cancer.
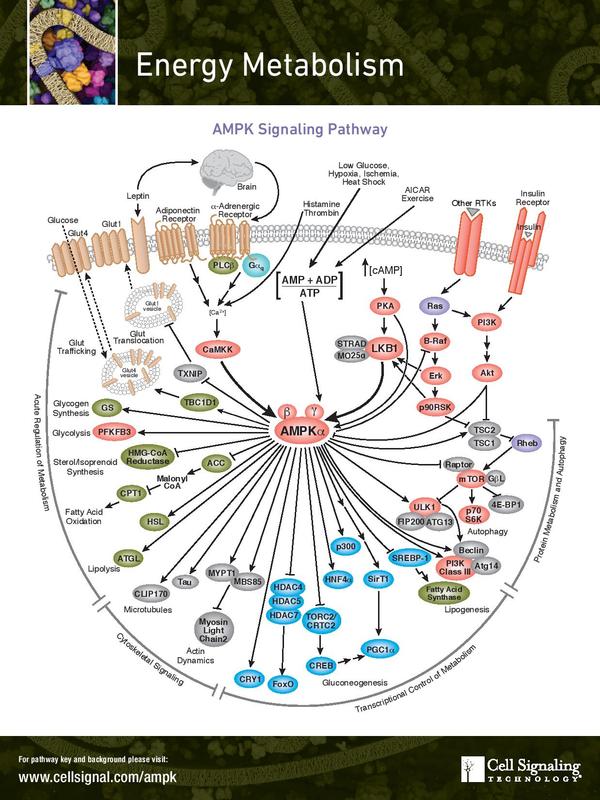
Keep an eye out in the coming weeks for "What the Hell? - Cancer". And just for fun I wanted to include what an actual signalling pathway looks like. Figure 6 shows the AMPK signalling pathway. AMPK regulates growth pathways and is commonly lost or down regulated in cancer. This, you will be shocked and appalled to know, is a pretty simple straight forward signalling pathway.
So you should all know the basics of genetics now! If you don’t read my blog post “What the Hell? - Genetics”.
To re-cap, your genetic code is held in a library. Each book is a gene. When the gene is “read” this creates RNA and the RNA can be translated into a protein. Genes/books can be coding genes or non-coding DNA. Your genes are made up of four letters in different sequences (A, T, G, C). You also have different sections in your library depending on the function of your cells e.g. brain, liver, skin. As I’ve mentioned before, key players in your genetic code being translated into a protein are the readers or “transcription factors”. But like any library you need some way of controlling all the pesky people running around. If transcription factors could pick up any book they want as many times as they wanted you get complete chaos. This happens in cancer where transcription factors get a hold of books they shouldn’t (called oncogenes) leading to cancer cells growing uncontrollably. The “people” that control this are your librarians also known as your “epigenome”. They control what books you can access, when you can take them out and how long you can take them out for. What is epigenetics? Epigenetics by definition is any act that alters gene expression without altering the genetic code. There’s a lot more to epigenetics than just controlling gene expression but for now we’ll just discuss this area.
I’m going to talk about two main branches or epigenetics – Histone post-translational modifications and DNA methylation.
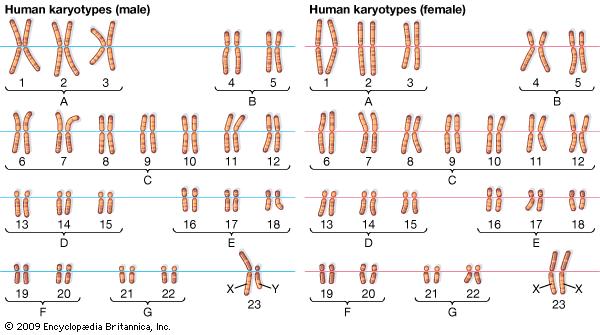
You have 22 pairs of normal chromosomes and 1 pair of sex chromosomes (XX or XY). The chromosomes are numbered by their size, where the largest pair is number 1 and the smallest pair is number 22 (see picture).
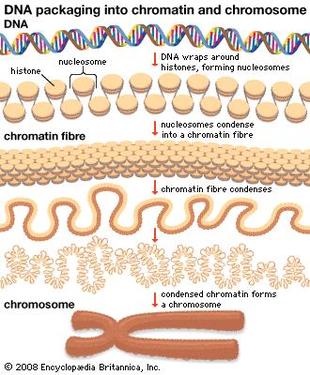
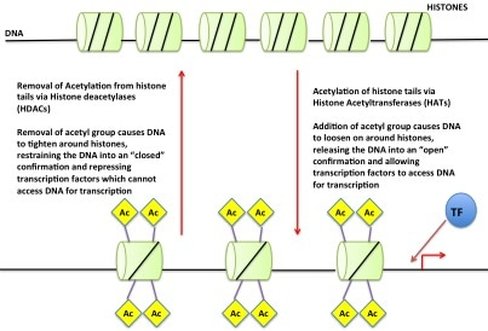
So we have our DNA in our nucleus as chromosomes but if all of your DNA is packaged so tight how do the readers get access? Little markers are placed on the histones, which tell the DNA to be open or closed. If the marks say “open” then the DNA unwinds from the histones allowing the readers to have access to the gene(s). An example of this (see picture) is called "Acetylation". An acetyl mark is added to histones. This tells the DNA to unwind, giving access to transcription factors. When the acetyl mark is removed the DNA closes up again, repressing gene expression.
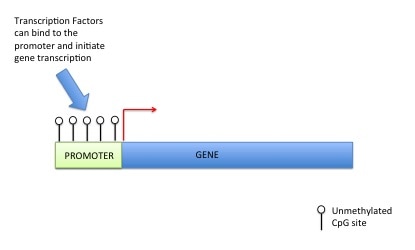
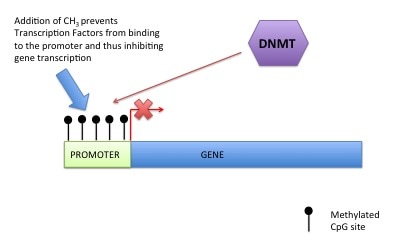
DNA methylation can also control gene expression. As I've told you before your genetic code is made up of four letters: A (Adenosine), T (Thymine), G (Guanine), C (Cytosine). DNA methylation happens at a C which has to be beside a G (i.e. CG). This is called a CpG site (the "p" means beside or 5' to). A methyl group is added onto the C (or cytosine). This prevents transcription factors or readers getting access to DNA, preventing gene expression (see pictures). For those that are interested a methyl group is a carbon with three hydrogens (CH3). The methyl group is put on by a DNA methyltransferase (this is an ezyme).
For those less interested, imagine you are trying to get access to a fridge. If the door is unlocked you can get in and eat but if the door is locked you can't get in. No food for you mister!
But why is this important?
Beside the fact my entire PhD is based on epigenetics...... Back to the library. We've talked before about wanting our cells to stay the way they are. No one wants a liver cell in their eye. Epiegenetics controls this. The librarians tell the readers what books they can access. They create the sections of books for different cell types and the barriers to prevent skin cells from acting like stomach cells (you'd excrete a lot of acid and mucus from your arms...gross). They also control how many times you can access the book - if you want a book to only be read once or all the time (house keeping genes) and how many copies of the book you take out. This is important for many diseases especially for cancer. In each cell you have two copies of a gene. Usually you only need one copy to be functional to live. But if your cells decide to express both copies this could lead to massive changes in the growth of the cell. For example, there is a gene called IGF2 which is important in growth and development. But we only want ONE copy of this gene. Two copies would be like super sizing all your meals. You are going to get very fat very quickly. IGF2 is one of these "oncogenes" I mentioned earlier. Loss of epigenetic marks around the second copy of this gene can lead to massive increases in tumour cell growth. Cancer is a very clever reader and can trick librarians. It can cause librarians to be distracted, allowing the cancer cell access to genes it shouldn't be allowed near (this can be loss of DNA methylation or hypomethylation) OR it can make the librarians super strict and shut down access to genes a normal cell would have access to (this can be gain of methylation or hypermethylation). Hypomethylation tends to happen to genes that will help cancer cells while hypermethylation tends to happen to genes that will hinder cancer cells. There are other areas of epigenetics I haven't covered, like microRNAs and also the other roles of epigenetic modifications besides gene expression control. For now this is just a brief introduction to get you familiar with the basic ideas of epigenetics. If you have any questions or want more detail please don't hesitate to contact me!!
You are a miracle. Not in the sense of “it’s a miracle you got this far” (though this applies to some of us) but you are a scientific miracle. Inside each of your cells, one of the smallest parts that make up “you”, is an extraordinary amount of information. Information we have barely begun to scratch the surface of understanding. Not only does each cell contain a small community of builders, energy makers, decision makers etc. but this community talks to other communities across vast distances to create organs, muscle, bone and in essence create you.
But how do these cells know who they are and what to do? How does a brain cell know it’s a brain cell and not a skin cell or a liver cell? How do they know their function in creating and maintaining you? This is down to your genes and the study of your genes is called genetics. Your cells are communities and have all the “buildings” you would have in a town (power supply, manufacturers etc.). The most important building in your cell is the library. The library contains all of the knowledge about you and your ancestors for millions of years. Like with any book editions change, books get updated and books get lost but the information is still there for you to access. 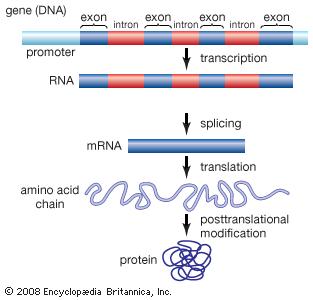 Transcription and Translation. Photo Credit: Encyclopaedia Britannica Inc. Britannica Inc, E., 2008. DNA. Available at: https://global.britannica.com/science/DNA [Accessed January 10, 2017] Transcription and Translation. Photo Credit: Encyclopaedia Britannica Inc. Britannica Inc, E., 2008. DNA. Available at: https://global.britannica.com/science/DNA [Accessed January 10, 2017]
As you know libraries contain books. Some books are very important and are read daily (for example the dictionary) while some books are only read when needed (for example a book on first aid) and lastly we have the books that hold very little useful information (for example trashy novels you read on holiday). The books are your genetic code and there is a lot of it. In fact if you took all the genetic information in an individual cell and laid it out like string it would measure 1 meter.
A gene is a book that creates an action. When you read a book you form an opinion on it and that opinion creates an idea, which you can act upon or share. When a gene is “read” this leads to the creation of an “idea” or RNA. You can act upon this “idea”, which leads to an “action” or makes a Protein. Proteins are used for everything in a cell, from builders, communication and regulation of the cells internal environment (temperature, amount of water, pressure etc.). For example you want to build a table so you read a book (gene) about carpentry. From that book you develop an idea (RNA) of how you want to build your table and copy it out in your own words. You then build your table (protein). That table can now be used however you want. This process is called transcription (genes to RNA) and translation (RNA to Protein). The books that are read daily are called “housekeeping” genes. These keep your cells working on the most basic levels (food, water, energy). The useful genes are called “coding genes”. This means that “reading” these will create RNA and protein(s). You have about 20,000 “coding genes” in your library. The final part of your genetic code is “junk DNA”. These are the books that cannot create RNA or protein(s). The books in every single cell in your body are identical. But your cells don’t all act the same. This is because certain cells only allow access to certain books. Imagine your library is divided into sections. For simplicity, the sections are your organs (e.g. brain, liver, skin) as well your blood and your immune system. If a cell is a brain cell it will only have access to the books in the brain cell section. The other sections are closed off. The only cells that have complete access to your entire library are “stem cells”. Stem cells are cells that can become any cell it wants. They are the roots of the tree from which all other cells come from. Once a cell becomes specialized (e.g. brain, liver or skin) it cannot be any other cell. Genes, like books, are made up of a series of letters. There are four letters in your genetic code: A (Adenosine), T (Thymine), G (Guanine) and C (Cytosine). Thousands of these letters are organized into specific patterns (e.g. AAAGCGTTCGAACG – this is not a real gene). The pattern is read like a book and converted into a specific RNA and protein. Interestingly, these books can be read forwards and backwards. They can also be cut up into sections (like a trilogy). Each different way of reading the book creates a different “idea” and “action”. However, there is always a beginning, middle and end. But who reads these books? The readers are called "transcription factors". They go into the libraries catalog and are sent to the book(s) they want. They transcribe the gene into RNA and pass it over to "workers" called "ribosomes" which convert the RNA code into protein. Neat! Different books are also read together. For example reading a French book with a French dictionary. One requires the other. While single books are read for simple ideas (for example eye colour or hair colour), multiple books are read for complex ideas (for example height). While you are created from your genes, your genes don’t determine everything about you. It’s important to keep in mind that your environment has a vital role to play in forming you. What you eat and drink, what pollutants you’re exposed to as well as what pathogens you’re exposed to all have an impact on you. You are a miracle. This is a basic introduction into genetics. As you can imagine this is a massive, complex area of study. If you have any questions or if you want more detail please don't hesitate to contact me! |
AuthorMy name is Caitriona and I am a PhD student at Imperial College London, UK. Categories
All
|